Gold - A Universal Symbol

Wherever an object is used to symbolize ultimate power, or status, whether religious, royal or governmental,
only one metal is deemed suitable; gold. This metal has always held a supreme place in Man’s regard.
The desire for gold has dominated most civilisations from time immemorial; it has shaped whole nations and
channeled the course of history, and is still today the cornerstone of economic life in the civilised world.
Early man soon discovered that nuggets of gold could easily be welded together by hammering and then beaten
into shape, with the gold retaining its strength and flexibility even when formed into thin fragile sheets
and shaped into domestic utensils. Its malleability (ability to bend, or be hammered, into new shapes) and
ductility (ability to stretch into a thin thread, or foil, without breaking) are such that one troy ounce of
gold (31.1035 grams) can be beaten into foil 10 m2 in area, a feature which has been used to beautiful effect,
for example, on the exquisite golden cupola domes of eastern mosques, cathedrals and temples.
Gold - Its Attributes

Gold has an extreme density (19.32 kg/cc); only platinum (21.45) of the well-known metals, is heavier, and lead (11.35) is substantially lighter. Its density allows easy accumulations of wealth, which gives security to its owner. It is easier to hoard gold than any other form of wealth.
Another attribute which ensures that gold is regarded as one of the most precious metals is its scarcity. In terms of volume, all the gold ever mined to date would still only occupy a cube with sides some 16m, weighing about 90,000 tonnes, or about 3,000 million troy ounces. In terms of scientific fact, gold is much less common than all the so-called Rare-Earth Elements, such as thulium, samarium, dysprosium, holmium and europium. Of the naturally occurring elements, only tellurium and some platinum group elements are less abundant than gold in the earth’s crust.
The attractive colour and lustre of gold are permanent features, as it is chemically inert and therefore resistant to oxidation and corrosion by virtually all naturally occurring substances. This incorruptible image has become a symbol for enduring power and wealth in a world where most things tarnish and decay. This superior durability of gold makes it the most “noble” of all the precious metals.
Its physical properties make gold a totally recyclable metal and, in addition to the supply of gold from new production every year, a proportion of gold coming onto the market comes from melting down existing objects, coins and jewelry. No gold is ever purposely discarded.
Hallmark Gold

If any trust was to be placed in objects made from gold, people needed to know exactly how much gold was in alloy, and the British system of hallmarking gold and silver (the most rigorous of any country) is known to have existed long before the Worshipful Company of Goldsmiths obtained their Charter in 1327 (the word “hallmark” denotes the mark put on an object at “Goldsmiths” hall).
Gold Carats

In jewellery, the term used to express the fineness of gold is the carat, or karat. This word comes from the Arabic “kirat”, meaning the seed of the locust-bean-tree. These seeds are remarkably uniform in weight, and hence formed the basis of the weighing system for gold among African and Arab traders. Nowadays, when applied to gold, a carat is no longer a unit of weight, but it means a twenty-fourth part. Thus, 18 carat gold contains 18 parts of pure gold, and 6 parts of alloy, in the rest of the industry, the fineness of different “caratages” is shown in the following table:
|
CARATAGES OF GOLD |
|
Caratage |
24 |
22 |
21 |
18 |
14 |
10 |
9 |
8 |
|
Fineness |
999 |
916 |
875 |
750 |
585 |
417 |
375 |
333 |
|
% Gold |
99.9% |
92.6% |
87.5% |
75.0% |
58.5% |
41.7% |
37.5% |
33.3% |
Colored Gold

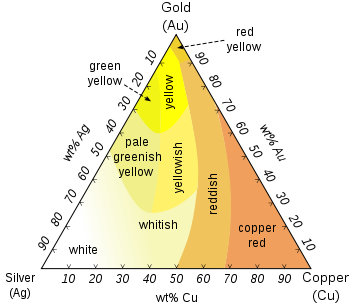
While pure gold is yellow in color, colored gold can be developed into various colors. These colors are generally obtained by alloying gold with other elements in various proportions.
For example, alloys which are mixed 14 parts gold to 10 parts alloy create 14-karat gold, 18 parts gold to 6 parts alloy creates 18 karat, and so on. This is often expressed as the result of the ratio, i.e.: 14/24 equals 0.585 and 18/24 is 0.750. There are hundreds of possible alloys and mixtures, but in general the addition of silver will color gold white, and the addition of copper will color it red. A mix of around 50/50 copper and silver gives the range of yellow gold alloys the public is accustomed to seeing in the marketplace. A small amount (0.2%) of zinc can be added to harden the alloy.
The most common grades of gold, in addition to pure 24K, are 22K (92%), 18K (75%), 14K (58%), 10k (41%) and 9K (38%). Colored golds can be classified to three groups:
• The Au-Ag-Cu system, producing white, yellow, green and red golds; typically malleable alloys.
• The intermetallic compounds, producing blue and purple golds, as well as other colors. These are typically brittle but can be used as gems and inlays.
• The surface oxide layers, such as black gold; mechanical properties depend on the bulk alloy, and the colored surface is prone to wear.
|
|
White Gold
|
White gold is an alloy of gold and at least one white metal, usually nickel, manganese or palladium. Like yellow gold, the purity of white gold is given in karats.
White gold's properties vary depending on the metals and proportions used. As a result, white gold alloys can be used for many different purposes; while a nickel alloy is hard and strong and therefore good for rings and pins, gold-palladium alloys are soft, pliable and good for white gold gemstone settings, sometimes with other metals like copper, silver, and platinum for weight and durability, although this often requires specialized goldsmiths. The term white gold is used very loosely in the industry to describe karat gold alloys with a whitish hue. Many believe that the color of the rhodium plating, which is seen on many commercial pieces, is actually the color of white gold. The term "white" covers a large spectrum of colors that borders or overlaps pale yellow, tinted brown, and even very pale rose. The jewelry industry often improves these off-white colors by rhodium plating.
|

|
A common white gold formulation consists of 90 wt.% gold and 10 wt.% nickel. Copper can be added to increase malleability.
The strength of gold-nickel-copper alloys is caused by formation of two phases, a gold-rich Au-Cu, and a nickel-rich Ni-Cu, and the resulting hardening of the material.
The alloys used in jewelry industry are gold-palladium-silver and gold-nickel-copper-zinc. Palladium and nickel act as primary bleaching agents for gold; zinc acts as a secondary bleaching agent to attenuate the color of copper.
Contact Allergy
About one out of eight people has an allergic reaction to the nickel in some white gold alloys when worn over long periods. A typical reaction is a minor skin rash. Because of this, many European countries do not use nickel white gold. White gold alloys made without nickel are less likely to be allergenic.
|
Rose, Red, and Pink Gold
|
The highest karat version of rose gold is also known as crown gold, which is 22 karat. Eighteen karat red gold may be made of 25% copper and 75% gold. For 18 karat rose gold, typically about 4% silver is added to 75% gold and 21% copper to give a rose color. 14 karat red gold is often found in the Middle East and contains 41.67% copper.
Rose gold in musical instruments
High-end flutes are very commonly made of solid rose gold, the most common alloy being 14K, but 9K, 10K, 18K and 19.5K are also available from the major flute makers.
|
Spangold
|
Some gold copper-aluminum alloys form a fine surface texture at heat treatment, yielding an interesting spangling effect. At cooling, they undergo a quasi-martensitic transformation from body centered cubic to body-centered tetragonal phase; the transformation does not depend on the cooling rate. A polished object is heated in hot oil to 150 - 200°C for 10 minutes then cooled below 20°C, forming a sparkly surface covered with tiny facets.
The alloy of 76% gold, 19% copper, and 5% aluminum yields yellow color, the alloy of 76% gold, 18% copper and 6% aluminum is pink.
|
Green Gold
|
Green gold alloys are made by leaving the copper out of the alloy mixture and just using gold and silver. It actually appears as a greenish yellow rather than green. Eighteen karat green gold would therefore contain a mix of gold 75% and silver 25% (or 73% gold and 27% silver). Fired enamels adhere better to these alloys.
Green gold was known to Lydians as long ago as 860 BC under the name electrum, a naturally occurring alloy of silver and gold.
Cadmium can be added to gold alloys in amount of up to 4% to achieve green color. The alloy of 75% gold, 23% copper, and 2% cadmium yields light green 18ct gold. The alloy of 75% gold, 15% silver, 6% copper, and 4% cadmium yields a dark green alloy. Cadmium is, however, toxic.
|
Grey Gold
|
Grey gold alloys are made by adding silver, manganese and copper in specific ratios to the gold.
|
Black Gold
|
Black gold is a type of gold used in jewelry. Black colored gold can be produced by various methods:
• Electroplating, using black rhodium or ruthenium. Solutions that contain ruthenium give a slightly harder black coating than those that contain rhodium.
• Patination by applying sulfur and oxygen containing compounds.
• Plasma assisted chemical vapor deposition process involving amorphous carbon.
• Controlled oxidation of gold containing chromium or cobalt (e.g. 75% gold, 25% cobalt).
A range of colors from brown to black can be achieved on copper-rich alloys by treatment with potassium sulfide.
Cobalt-containing alloys, e.g. 75% gold with 25% cobalt, form a black oxide layer with heat treatment at 700-950 °C. Copper, iron and titanium can be also used for such effect. Gold-cobalt-chromium alloy (75% gold, 15% cobalt, 10% chromium) yields surface oxide that's olive-tinted because of the chromium(III) oxide content, is about 5 times thinner than Au-Co and has significantly better wear resistance. The gold-cobalt alloy consists of a gold-rich (about 94% Au) and cobalt-rich (about 90% Co) phases; the cobalt-rich phase grains are capable of oxide layer formation on their surface.
More recently a laser technique has been developed that renders the surface of metals deep black. A femtosecond laser pulse deforms the surface of the metal forming nanostructures. The immensely increased surface area can absorb virtually all the light that falls on it thus rendering it deep black.
|
Purple and Blue Gold
|
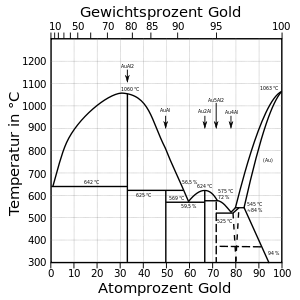
Purple gold (also called amethyst gold and violet gold) is an alloy of gold and aluminum rich in gold-aluminium intermetallic (AuAl2). Gold content in AuAl2 is around 79% and can therefore be referred to as 18 karat gold. Purple gold is more brittle than other gold alloys, as it is an intermetallic compound instead of a malleable alloy, and a sharp blow may cause it to shatter. It is therefore usually machined and faceted to be used as a "gem" in conventional jewelry rather than by itself. At lower content of gold, the material is composed of the intermetallic and an aluminium-rich solid solution phase. At higher content of gold, the gold-richer intermetallic AuAl forms; the purple color is preserved to about 15% of aluminium. At 88% of gold the material is composed of AuAl and changes color. (The actual composition of AuAl2 is closer to Al11Au6 as the sublattice is incompletely occupied.)
Blue gold is an alloy of gold and indium. It contains 46% gold (about 12 ct) and 54% indium, forming an intermetallic compound AuIn2. While several sources remark this intermetallic to have "a clear blue color," in fact the effect is slight: AuIn2 has CIE LAB color coordinates of 79, -3.7, -4.2 which appears roughly as a greyish color. With gallium, gold forms an intermetallic AuGa2 (58.5% Au, 14ct) which has slighter bluish hue. The melting point of AuIn2 is 541 °C, for AuGa2 it is 492 °C. AuIn2 is less brittle than AuGa2, which itself is less brittle than AuAl2.
All the AuX2 intermetallics have crystal structure of CaF2 and therefore are brittle. Deviation from the stoichiometry results in loss of color. Slightly nonstoichiometric compositions are however used, to achieve a fine-grained two- or three-phase microstructure with reduced brittleness. A small amount of palladium, copper or silver can be added to achieve a less brittle microstructure.
|
|
The intermetallic compounds tend to have poor corrosion resistance. The less noble elements are leached to the environment, and a gold-rich surface layer is formed. Direct contact of blue and purple gold elements with skin should be avoided as exposition to sweat may result in metal leaching and discoloration of the metal surface.
A surface plating of blue gold on karat gold or sterling silver can be achieved by a gold plating of the surface, followed by indium plating, with layer thickness matching the 1:2 atomic ratio. A heat treatment then causes interdiffusion of the metals and formation of the required intermetallic compound.
Oxide Layer Blue Gold
Blue gold can be achieved by formation of an oxide layer on an alloy of 75% gold, 24.4% iron, and 0.6% nickel; the layer forms on heat treatment in air between 450–600 °C.
Gold of purity 20–23 carat, when alloyed with ruthenium, rhodium and three other elements and heat-treated at 1800 °C, forms a 3–6 micrometers thick surface layer with a rich sapphire blue color.
|
|